CIP and SIP systems for horizontal scraper centrifuges
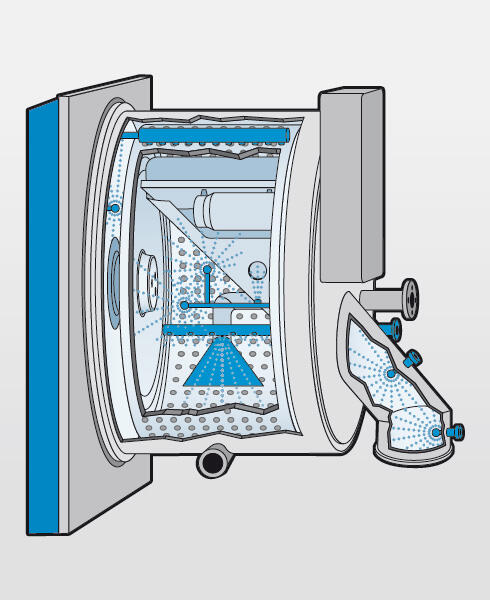
Pure and clean
CIP system
For cleaning the centrifuge process area, a CIP system (Cleaning In Place) can be integrated. This system is used during a product or batch change to eliminate the risk of cross-contamination. The CIP nozz les, the feed and wash system as well as the residual heel removal outside the basket clean the process area.
SIP system
After the CIP cleaning, SIP cleaning (Sterilisation In Place) can be undertaken. To eliminate microorganisms, the process area is wetted with disinfectant (e.g. hydrogen peroxide, sodium hydroxide, etc.) via the CIP system.
Partial flooding of the process area
The process area can also be partially flooded. By changing the speed and direction of rotation of the basket, a «washing machine effect» is achieved, which contributes to effective cleaning of the process area.
GMP design for efficient cleaning
Our designs comply with the latest GMP guidelines. The hygenic cleaning of the process area is made possible by a clean finish, excellent surface quality, compliance with minimum radii and the use of FDA-approved open O-rings.
Low solvent consumption
The compact design as well as optimised cleaning programs ensure efficient cleaning with low solvent consumption.

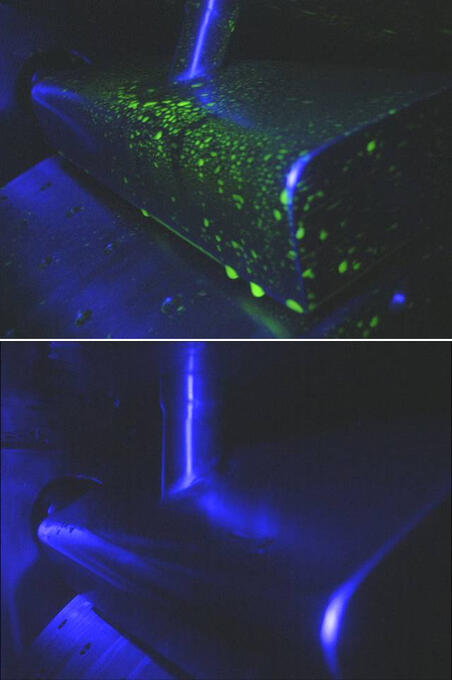
Riboflavin tests
Verifiable cleanliness
We optimise the CIP programs for the different centrifuge types with the aid of riboflavin tests. This way it is ensured that even with low solvent consumption, all surfaces in the process area are wetted with cleaning liquid. Riboflavin tests will be demonstrated on request during the FAT (Factory Acceptance Test).

Brochures
Download matching brochures for the product as PDF here.

Your contact person
Find the right contact person for your needs at Ferrum, our agencies and locations.
We look forward to hearing from you.
